CHEST-eR Reanimation Device
CHEST-eR Reanimation Device
Supplied with 2 protection sheaths - Warranty 1 year
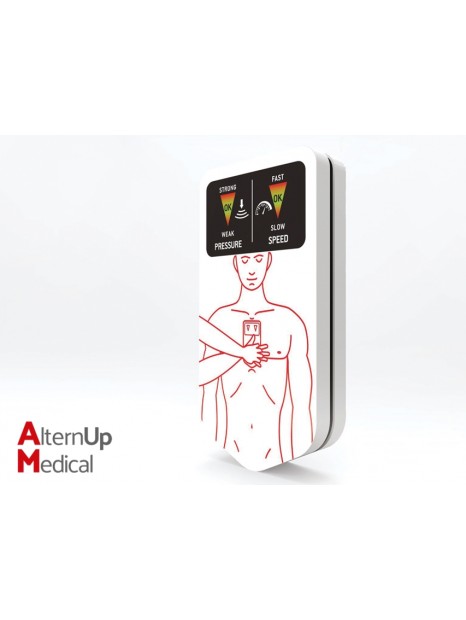
Chest-eR is a new generation device which assists and improves cardiac massage practice and can be used with defibrillators.
It can be used by rescuers, hospital staff, common people and educators. It has been designed to reduce the incidence of internal injuries, providing protection during chest compressions.
A special 3 layer internal structure helps reducing dangerous stresses produced by incorrect application of force, redistributing excessive forces and guaranteeing hygiene.
The device includes luminous indicators that inform and show the rescuer on correct pressure and compression rate.

Worldwide Delivery

After Sales Service Guaranteed

Secured Payment
CHEST-eR Reanimation Device
Supplied with 2 protection sheaths - Warranty 1 year
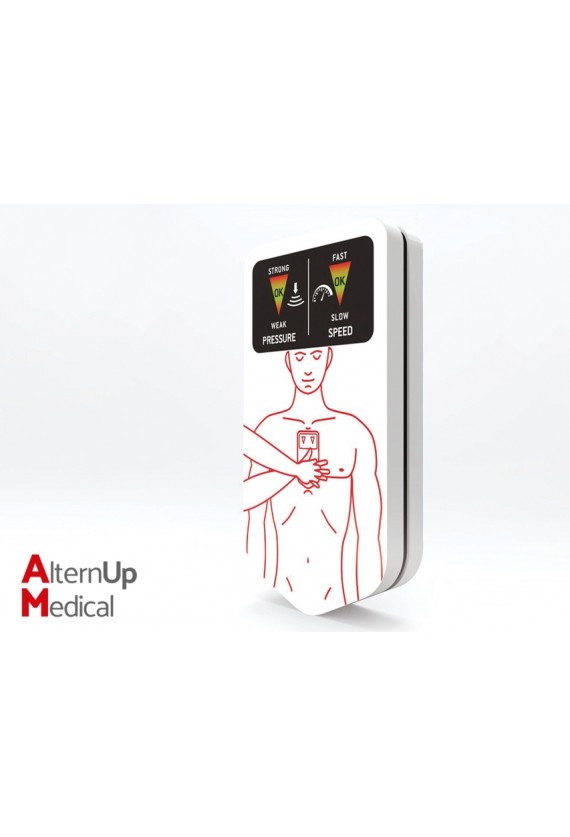
Chest-eR is a new generation device which assists and improves cardiac massage practice.
It can be used by rescuers, hospital staff, common people and educators. It has been designed to reduce the incidence of internal injuries, providing protection during chest compressions.
A special 3 layer internal structure helps reducing dangerous stresses produced by incorrect application of force, redistributing excessive forces and guaranteeing hygiene.
The device includes luminous indicators that inform and show the rescuer on correct pressure and compression rate.
The device perfectly combines with manikins updated according to International Guidelines (i.e. Laerdal Medical, Brayden, ......) and can be used with defibrillators.
Features:
- intuitive and easy-to-use
- compact and lightweight, used with 1.5V battery
- proper resuscitation in complete safety
- ensures maximum effectiveness of cardiac massage
- non-Newtonian materials dissipates impact of too violent compressions absorbing up to 90% of energy
Medical Device Class I.
Specific References
11 other products in the same category:
Interested in product
Tell us your interest in this product and we will contact you for more details.